
|
|
 |
Laser DMR | |||||||||||||||||||||||||||||||||||||||||||||
| Ischemic coronary disease is the leading cause of morbidity and mortality in the Western world. Most available therapeutic approaches aim either at relieving symptoms by reducing myocardial oxygen demand, preventing further disease progression by modifying risk factors, restoring flow to a localized segment of the arterial tree (angioplasty or bypass surgery). However, it is becoming increasingly clear that a significant proportion of patients with ischemic heart disease are either suboptimal candidates for CABG/PTCA or receive incomplete revascularization with CABG/PTCA. Although this number is yet not well defined, it is estimated that up to 20-37% of patients with ischemic heart disease are suboptimal candidates for either standard revascularization strategy. Furthermore, a significant proportion of these patients have persistent symptoms of angina despite maximal medical therapy. Therefore, an alternative "revascularization" strategy is needed in these patients that would not only relieve their angina and improve their quality of life, but would also result in an improvement in left ventricular function and myocardial perfusion. Laser myocardial revascularization may serve that role. (See Conclusion at the end of the text) | |||||||||||||||||||||||||||||||||||||||||||||||
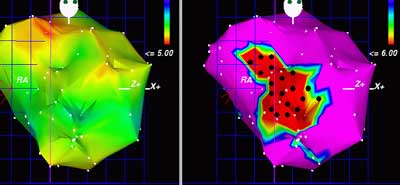
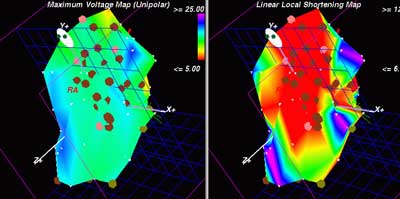

Historical Prespective: From the earlier and obsolete
use of mechanical myocardial revascularization using needle puncture and left
ventricular biopsy in the 60's, the use of CO2 laser in the 80's initially during
CABG and subsequently as a stand alone procedure using lateral thoracotomy,
to the introduction of percutaneous laser myocardial revascularization in the
late 90's, a recurring theme was noted: All these procedures resulted in a significant
improvement in angina class and exercise capacity without any consistent demonstrable
improvement in left ventricular function or myocardial blood flow. The concept,
Transmyocardial Laser Revascularization, was based on the work of early investigators
who were seeking to emulate reptilian circulation in the mammalian heart. A
variety of techniques which included needle acupuncture, punch biopsy boring
of left ventricular conduits, and insertion of T-Tubes into the left ventricle
were attempted. Initial results, in the first one to two months, were promising.
The mechanical injury, initiated the sequence of cell infiltration, fibrosis,
scarring, and closure of these channels after two months. A study of what was
then a new source of energy, the laser, which was developed in the mid 1960's
by Maimon, and an interest in the work of the early investigators led to the
initial animal studies that showed that following coronary ligation infarct
size was smaller in hearts that were revascularized by laser, mortality was
reduced, and channels remained patent (results first presented in 1971). In
the early 80's (1982), one patient, who could not be weaned from cardiopulmonary
bypass following coronary artery bypass grafting, was revascularized using the
laser and subsequently successfully weaned from bypass. That patient had an
uneventful postoperative course, 12 more patients were studied (adjunct to CABG),
with "incredible" anecdotal reports.
Development of the clinical high power CO2 laser system, the Heart Laser, for
transmyocardial laser revascularization on the beating heart by Laser Engineering,
now PLC Medical Systems, led to the first FDA approved clinical trials as a
stand alone therapy in the United States.
Clinical LMR data: Adjunctive (to CABG) LMR was performed in 12 Patients with
incomplete revascularization by CABG; None of the patients died with a follow-up
ranging from 3-32 months. Postoperative thallium stress tests and left ventriculography
"reportedly"?? indicated that channels remained patent and perfused
the myocardium.61 Severeal case reports and small case series followed claiming
improvement in angina and myocardial perfusion, as well and patency of the laser
channels. Surgical LMR was used as sole therapy in 21 patients. Eight patients
were excluded from follow-up because of death (n=5), rerevascularization (n=2),
or diaphragmatic paralysis resulting in postoperative respiratory incapacity
(n=1). In the remaining 13 patients available for 12-month follow-up, angina
class (Canadian Cardiovascular Society) decreased from 3.7±0.4 to 1.8±0.6,
resting mean subendocardial/subepicardial perfusion ratio increased by 20±9%
in septal regions treated by laser but decreased by 2±5% in untreated
regions (n=11, p <0.001). Surgical LMR (CO2) was performed in 20 patients,
5 patients died (2 in-hospital). There was an improvement in angina class and
?? nuclear defect size. Surgical LMR (CO2) was performed in 61 patients at Hamburg
University and improved clinical status in 50 of 61 treated patients and 6 patients
(9.8%) died. Histopathological investigations revealed tissue remodeling comparable
with different stages of wound healing. By 1997, over 3000 patients worldwide
have been treated with the CO2 laser. A nonrandomized phase II trial was completed
in 1995. A randomized controlled phase III trial has completed enrollment by
1997. In each trial 200 patients with endstage coronary artery disease and severe
disabling angina that was not amenable to conventional revascularization were
enrolled. Follow-up showed that 80% of patients showed a significant improvement
in angina class status postoperatively and 30% had no angina at one year of
follow-up. Nuclear data was less convincing but still showed some improvement.
The phase II non-randomized study enrolled 200 patients at eight U.S. hospitals.
Follow-up averaged 10±3 months. Perioperative mortality was 9%. Angina
class decreased significantly (p < 0.001) and there was a significant decrease
in the number of perfusion defects in the treated left ventricular free wall.
Results of the phase III randomized (open label) CO2 LMR study were similar
with less clear improvement on nuclear perfusion scans. Ho:Yag LMR was then
studied in a similar patient population and resulted in a similar improvement
in angina class with less dramatic (if any) improvement in nuclear scans.
The use of Holium:YAG LMR has enabled percutaneous laser myocardial revascularization,
which in phase II unblended studies showed an improvement in symptoms.
LMR mechanism of action: Although the mechanism of laser
myocardial revsacularization remains uncertain. The proposed mechanisms include:
1. Myocardial angiogenesis resulting from release of growth factors and inflammatory
mediators
2. Myocardial denervation leading to symptom control
3. Myocardial fibrosis resulting in a tethering action to improve myocardial
function and promote favorable remodelling
4. Perfusion through laser channels, a hypothesis that is becoming less likely
with the accumulation of histologic data

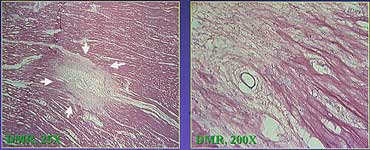
With the recent approval of the PLC Laser system,
Laser Myocardial Revascularization (LMR) was emerging as a viable option for
patients who are suboptimal candidates for standard revascularization strategies.
However, surgical LMR would be difficult to implement as a stand-alone treatment
strategy because it necessitates general anesthesia, "mini" thoracotomy,
and hospitalization. The development of percutaneous LMR strategies particularly
using the 3D Biosense navigation system has enabled LMR without the need for
general anesthesia, surgical access, or hospitalization, making percutaneous
LMR (PLMR), if effective, a more acceptable "revascularization" or
"antianginal" strategy.
A major unanswered question that will impact the use of PLMR in patients with
ischemic heart disease is whether LMR is indeed a revascularization strategy,
or does it only provide symptomatic relief without any effects on survival,
myocardial infarction, need for repeat revascularization, or left ventricular
function. Will LMR be used if it is not a revascularization strategy??? It is
becoming clear from registry data and from randomized placebo-controlled yet
unblinded studies that LMR results in a significant improvement in angina class,
quality of life, and exercise capacity. However, the unblinded nature of these
studies makes these conclusions partially attributable to placebo effect. What
these studies conclude, however, is that improvements in perfusion and myocardial
function with LMR are either not present or cannot be detected with currently
available imaging modalities. This might be secondary to the limitations of
currently applied imaging tests, including the poor spatial resolution of stress
nuclear perfusion scans, the poor edge detection and operator variability for
echocardiographic methods, and the inability of coronary angiography to visualize
a large portion of existing collaterals. It is clear that for LMR to become
mainstream therapy, its mechanism of action needs to be better elucidated using
more sensitive imaging modalities. Magnetic resonance imaging may serve that
role. Thus, the hypothesized angiogenic effect, if proven, may make laser myocardial
revascularization a more genuine "revascularization" strategy that
can be accepted by both patient and treating physician.
The question of the efficacy of laser DMR was finally answered by the DIRECT study of percutaneous laser myocardial revascularization which enrolled 300 patients randomized in a blinded fashion to laser myocardial revascularization or placebo. The 6 months data was clear in demonstrating that laser myocardial revascularization was not superior to medical therapy in these patients, suggesting that all the pervious benefits seen with this treatment were secondary to the placebo effect.