
|
|
 |
||||||||||||||||||||||||||||||||||||||||||||||
|
Preclinical studies
use a range of species and provide a comprehensive approach to the study
of drug effect and gene function in a variety of acute and chronic ischemia
models. The Center has extensive experience using the ameroid constrictor
porcine models and various other models of clinical angiogenesis. In fact,
preclinical studies performed at the Center provided the basis for two
of the largest angiogenesis trials to date (Genentech's VIVA VEGF study
and Chiron's FIRST FGF-2 study). The rapid evaluation of angiogenic agents
in small animal models has also enabled rapid proof of principle evaluation
and mechanistic insights by using knock-out models with selective restoration
of various genes. Finally, the Center's drug
delivery program allows the rapid assessment and optimization of novel
delivery catheters and vectors in various animal models
|
|||||||||||||||||||||||||||||||||||||||||||||||
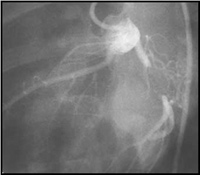 Ameroid Constrictor porcine
Model: Angiography shows total occlusion of the left circumflex artery, however,
with growth factor administration, both epicardial and intramyocardial collaterals
are seen with almost complete reconstitution of the distal circumflex artery
and absence of myocardial ischemia at rest. With stress, LCX flow remains lower
than normal flow (LAD), but is significantly higher than control animals. These
models are used to test the efficacy of various growth factors and gene therapy
vectors in inducing functionally significant angiogenesis. Researchers at the
Center have also been able to minimize the variability of the model by dedicated
surgeons and interventional cardiologists, dedicated tissue and image analysis
staff, and the use of multiple outcome measures including microspheres, histology,
magnetic resonance imaging, echocardiographic assessment, molecular biology
markers, and coronary casting studies
Ameroid Constrictor porcine
Model: Angiography shows total occlusion of the left circumflex artery, however,
with growth factor administration, both epicardial and intramyocardial collaterals
are seen with almost complete reconstitution of the distal circumflex artery
and absence of myocardial ischemia at rest. With stress, LCX flow remains lower
than normal flow (LAD), but is significantly higher than control animals. These
models are used to test the efficacy of various growth factors and gene therapy
vectors in inducing functionally significant angiogenesis. Researchers at the
Center have also been able to minimize the variability of the model by dedicated
surgeons and interventional cardiologists, dedicated tissue and image analysis
staff, and the use of multiple outcome measures including microspheres, histology,
magnetic resonance imaging, echocardiographic assessment, molecular biology
markers, and coronary casting studies
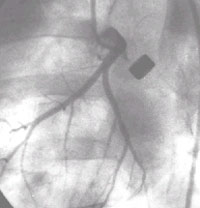 Ameroid Constrictor model:
Angiography shows the metal ameroid constrictor occluding the left circumflex
artery. This was a control animal with no filling of the distal vessel via collaterals
and severe ischemia of the LCX distribution even at rest. Although the results
for individual animals have a large variability with regards to the degree of
native collateralization and ischemia induced collateralization, the use of
8-10 animals per treatment group and the use of multiple outcome measures minimizes
the effect of this variability and allows a reproducible assessment the angiogenic
potential of candidate genes and cytokines. In addition, as is done in clinical
trials, all studies at the centers are randomized, vector-vehicle controlled,
and the investigators and assessors are blinded to treatment assignment eliminating
any potential bias
Ameroid Constrictor model:
Angiography shows the metal ameroid constrictor occluding the left circumflex
artery. This was a control animal with no filling of the distal vessel via collaterals
and severe ischemia of the LCX distribution even at rest. Although the results
for individual animals have a large variability with regards to the degree of
native collateralization and ischemia induced collateralization, the use of
8-10 animals per treatment group and the use of multiple outcome measures minimizes
the effect of this variability and allows a reproducible assessment the angiogenic
potential of candidate genes and cytokines. In addition, as is done in clinical
trials, all studies at the centers are randomized, vector-vehicle controlled,
and the investigators and assessors are blinded to treatment assignment eliminating
any potential bias
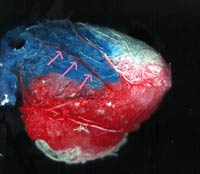 Ameroid Constrictor model
with vascular casting: Polymers with different colors are used (blue for LCX,
Red for LAD, White for RCA) and are injected in the coronary arteries. The polymers
will then be allowed to solidify and the myocardium is digested leaving the
vascular framework. In this image, arrows point to epicardial collaterals from
the LAD and RCA distributions to the ischemic LCX artery. This efficacy measure
is used in conjunction with all the other beforementioned measures to enable
an accurate asessment of the angiogenic potential of candidate genes and molecules.
Using this methods, we were able to document these epicardial collaterals (feeding
collaterals, arteriogenesis) which are essentials to successful revascularization
since they provide the major source of increased blood flow to the intramyocardial
collaterals
Ameroid Constrictor model
with vascular casting: Polymers with different colors are used (blue for LCX,
Red for LAD, White for RCA) and are injected in the coronary arteries. The polymers
will then be allowed to solidify and the myocardium is digested leaving the
vascular framework. In this image, arrows point to epicardial collaterals from
the LAD and RCA distributions to the ischemic LCX artery. This efficacy measure
is used in conjunction with all the other beforementioned measures to enable
an accurate asessment of the angiogenic potential of candidate genes and molecules.
Using this methods, we were able to document these epicardial collaterals (feeding
collaterals, arteriogenesis) which are essentials to successful revascularization
since they provide the major source of increased blood flow to the intramyocardial
collaterals