
|
|
 |
Cell Transplantation | |||||||||||||||||||||||||||||||||||||||||||||
|
The adult heart lacks repair
capabilities and cannot regenerate itself after myocardial injury (Myocardial
Infarction, Heart Attack). Thus patients often develop congestive heart
failure that may be fatal. Researchers at the Angiogenesis Research Center
are investigating the use of autologous cells transplantations to attempt
to regenerate the damaged heart. We biospy skeletal muscles (legs) and
isolate cells with regenerative potential, allow them to multiply, and
then reimplant the cells into areas of damaged myocardium to restore myocardial
function. In addition, pleuripotent cstem cells can be used to regenerateheart
muscle and specialized cells and cells can be used for the delivery of
transgenes to the heart (gene therapy) allowing regulatable gene expression
and multigene expression. Most Recently Autologous myotissue transplantation has been studied with remarkable preclinical success.
|
|||||||||||||||||||||||||||||||||||||||||||||||
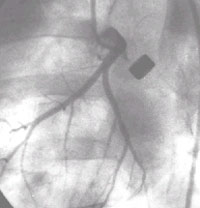 Ameroid Constrictor model:
Angiography shows the metal ameroid constrictor occluding the left circumflex
artery. This was a control animal with no filling of the distal vessel via collaterals
and severe ischemia of the LCX distribution even at rest. This would ultimately
result in transmural myocardial damage and infarction with scar formation. These
models can be used to test the regenerative potentials of several treatment
startegies including cell transplantation.
Ameroid Constrictor model:
Angiography shows the metal ameroid constrictor occluding the left circumflex
artery. This was a control animal with no filling of the distal vessel via collaterals
and severe ischemia of the LCX distribution even at rest. This would ultimately
result in transmural myocardial damage and infarction with scar formation. These
models can be used to test the regenerative potentials of several treatment
startegies including cell transplantation.
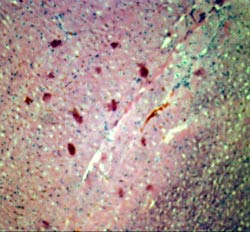 Autologous myoblast transplantation
was carried out in a canine model of myocardial infarction in an effort to investigate
myoblast survival and activity. Shown are histological section showing autologous
skeletal myoblast tranplanted successfully in the heart with short term survival
Autologous myoblast transplantation
was carried out in a canine model of myocardial infarction in an effort to investigate
myoblast survival and activity. Shown are histological section showing autologous
skeletal myoblast tranplanted successfully in the heart with short term survival
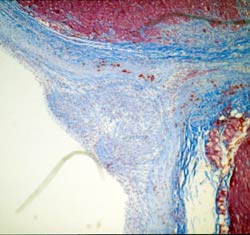 Delivery remains the achilles's
heal of many cellular transplantation approaches to angiogenesis and myogenesis.
Shown here is Trichrome staining of the needle track produced by intramyocardial
delivery emphasizing the fibrosis that results from the trauma and the paucity
of transplanted myoblasts that survive that injury
Delivery remains the achilles's
heal of many cellular transplantation approaches to angiogenesis and myogenesis.
Shown here is Trichrome staining of the needle track produced by intramyocardial
delivery emphasizing the fibrosis that results from the trauma and the paucity
of transplanted myoblasts that survive that injury
Cell-based gene transfer is a promising new strategy
that utilizes autologous cells transfected with a transgene of interest to express
that transgene in vivo. The advantage of such a system is to circumvent the
inflammatory response by using autologous cells and achieve prolonged expression
by stable transfection using various measures including in vitro retroviral
or lentiviral transfection. In addition, complex constructs can be performed
that would allow stable regulatable expression and multiple transgene expression.
To illustrate this delivery modality, endothelial cells were evaluated for their
potential use in gene transfer to deliver apolipoprotein E (apoE) in the murine
apoE knockout mice. After transplantation of the apoE secreting Pro-175 endothelial
cells into apoE-deficient mice, serum cholesterol levels were lower in animals
that have received the apoE secreting endothelial cells compared with the levels
of age-matched controls having received non-secreting endothelial cells. Concomitant
with cholesterol reduction, atherosclerotic aortic plaques were noticeably reduced
in the animals receiving apoE secreting endothelial cells. In other studies,
cell lines transduced with a retroviral vector containing the human erythropoeitin
(hEpo) cDNA driven by the hypoxia-responsive promoter. In vitro, these cells
showed a threefold increase in hEpo secretion as oxygen levels were shifted
from 21% to 1.3% oxygen. In animals treated with these altered cells, serum
hEpo levels in animals exposed to 7% oxygen were two-fold higher than values
seen in their control counterparts kept at 21% oxygen.
A discussion of the various delivery vectors would be incomplete without a discussion
of the specific needs for the cardiovascular system. Although sustained expression
would be desirable for cardiovascular diseases with a specific gene defect such
as familial hypercholesterolemia, various dystrophies or Lpa deficiency, transient
expression would be preferable for cardiovascular conditions that are self limited
such as restenosis after coronary angioplasty. In addition, therapeutic angiogenesis
would be best accomplished with a transient expression vector since sustained
expression of angiogenic cytokines may lead to pathological angiogenesis.